What to write
For each group, the numbers of participants who were randomly assigned, received intended intervention, and were analysed for the primary outcome
Examples
See Figure 1, Figure 2, and Figure 3.
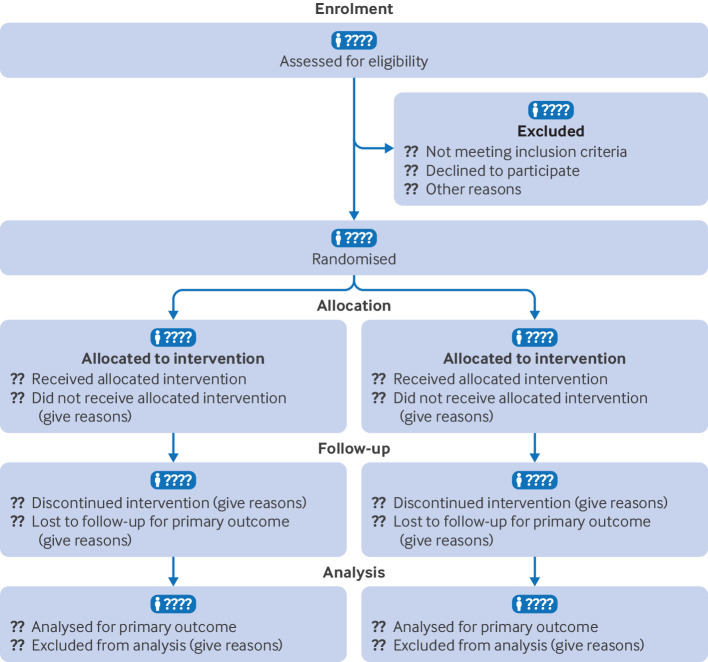
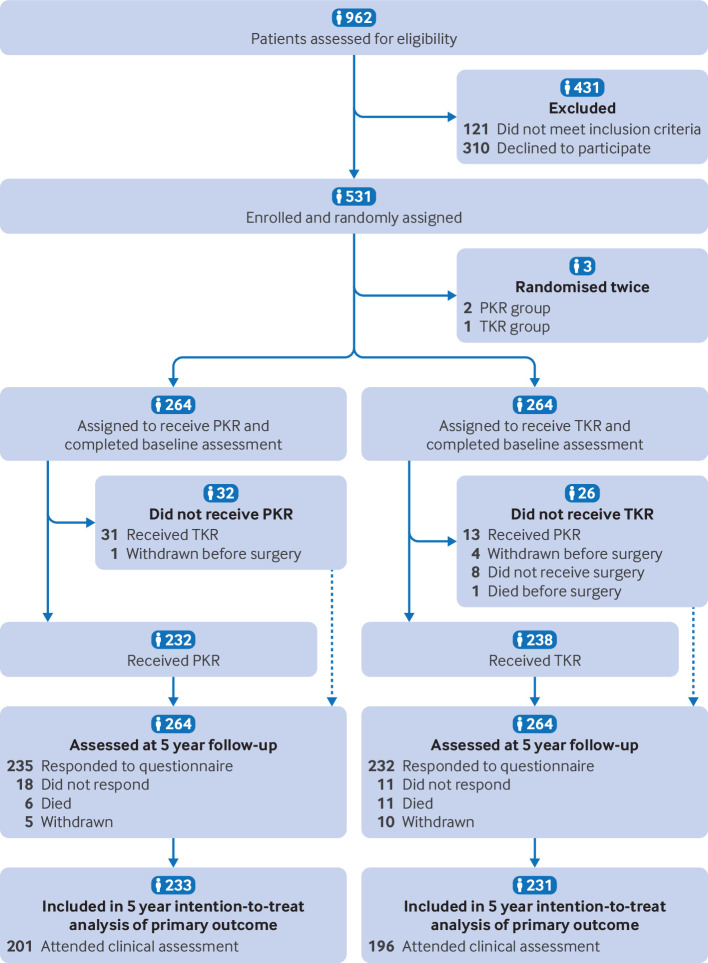
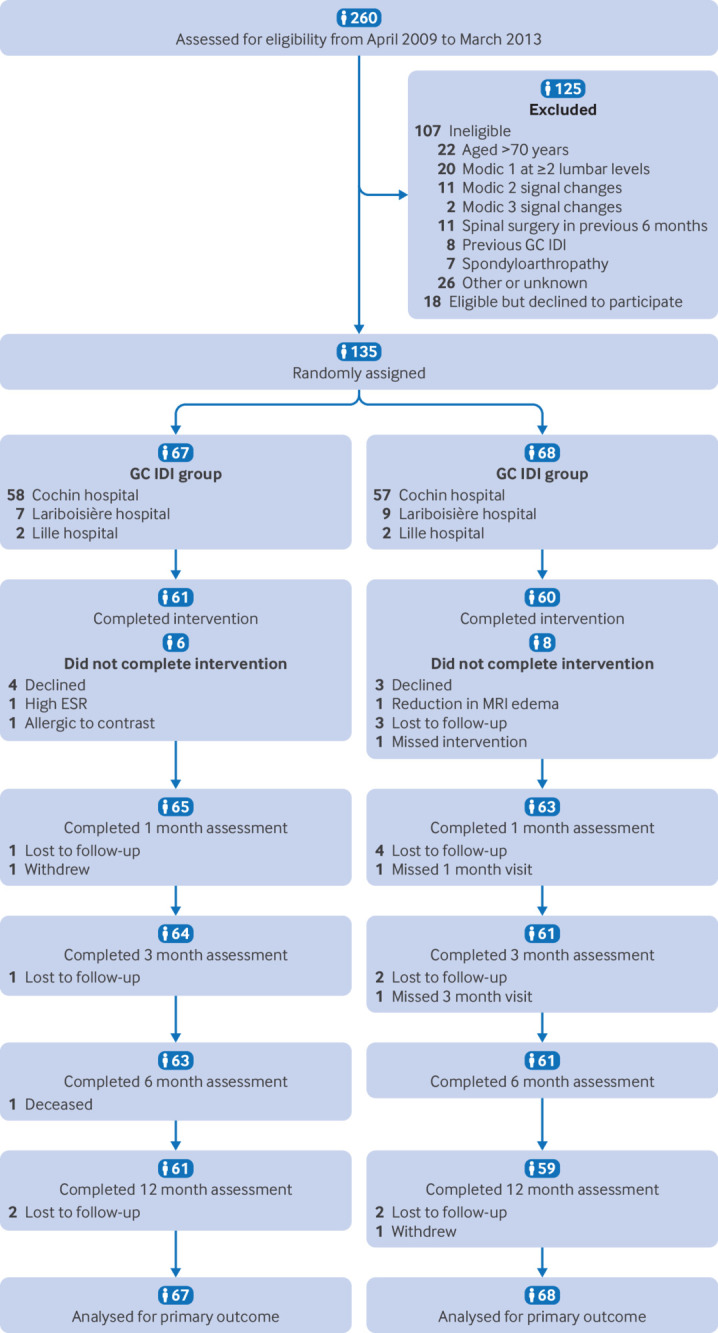
Explanation
The design and conduct of some randomised trials are straightforward, and the flow of participants, particularly where there are no losses to follow-up or exclusions, through each phase of the study can be described relatively easily. For other trials, it can be difficult for readers to discern whether and why some participants did not receive the treatment as allocated, were lost to follow-up, or were excluded from the analysis.3 This information is crucial for several reasons. Participants who were excluded after allocation are unlikely to be representative of all participants in the study. For example, participants may not be available for follow-up evaluation because they experienced an acute exacerbation of their illness or harms of treatment.4,5
Attrition as a result of loss to follow-up, which is often unavoidable, needs to be distinguished from investigator-determined exclusion for such reasons as ineligibility, withdrawal from treatment, and poor adherence to the trial protocol. Erroneous conclusions can be reached if participants are excluded from analysis, and imbalances in such omissions between groups may be especially indicative of bias.56,7 Information about whether the investigators included in the analysis all participants who underwent randomisation, in the groups to which they were originally allocated (item 21b), is therefore of particular importance. Knowing the number of participants who did not receive the intervention as allocated or did not complete treatment permits the reader to assess to what extent the estimated efficacy of therapy might be underestimated in comparison with ideal circumstances.
If available, the number of people assessed for eligibility, and reason for exclusion, should also be reported. Although this number is relevant to external validity only and is arguably less important than the other counts,8 it is a useful indicator of whether trial participants were likely to be representative of all eligible participants.
A suggested template for reporting the number of participants who were randomly assigned, received intended treatment, were lost to follow-up, and were analysed for the primary outcome is shown in figure 1, and the counts required are described in detail in Table 1. A review of randomised trials published in general medical journals found that reporting of what happened to participants and their data was considerably more thorough in articles that included a diagram of the flow of participants through a trial than in those that did not.3
| Stage | No of people included | No of people not included or excluded | Rationale |
|---|---|---|---|
| Enrolment | People evaluated for potential enrolment | People who did not meet the inclusion criteria or met the inclusion criteria but declined to be enrolled | These counts indicate whether trial participants were likely to be representative of all patients seen; they are relevant to assessment of external validity only, and they are often not available |
| Randomisation | Participants randomly assigned | — Cr | ucial count for defining trial size and assessing whether a trial has been analysed by intention-to-treat |
| Treatment allocation | Participants who received intervention as allocated, by trial group | Participants who did not receive intervention as allocated, by trial group | Important counts for assessment of internal validity and interpretation of results; reasons for not receiving intervention as allocated should be given |
| Follow-up | Participants who completed intervention as allocated, by trial group Participants who completed follow-up as planned, by trial group |
Participants who did not complete intervention as allocated, by trial group Participants who did not complete follow-up as planned, by trial group |
Important counts for assessment of internal validity and interpretation of results; reasons for not completing intervention or follow-up should be given |
| Analysis | Participants included in main analysis for the primary outcome, by trial group | Participants excluded from main analysis for the primary outcome, by trial group | Crucial count for assessing whether a trial has been analysed by intention-to-treat; reasons for excluding participants should be given |
Some information, such as the number of individuals assessed for eligibility, may not always be known,9 and depending on the nature of a trial, some counts may be more relevant than others. It will sometimes be useful or necessary to adapt the structure of the flow diagram to a particular trial. In some situations, other information may usefully be added. For example, for trials of non-pharmacological interventions it may be important to report the number of care providers or centres performing the intervention in each group and the number of participants treated by each care provider or in each centre.10
The exact form and content of the flow diagram may be varied according to specific features of a trial. For example, many trials of surgery or vaccination do not include the possibility of discontinuation. Although CONSORT strongly recommends using a flow diagram to communicate participant flow throughout the study, there is no specific, prescribed format.
Training
The UK EQUATOR Centre runs training on how to write using reporting guidelines.
Discuss this item
Visit this items’ discussion page to ask questions and give feedback.